swanson summer sweepstakes
south australia sex laws
bicycle sweepstakes 2017
all you can see and play sweepstakes
enter hgtv dream home sweepstakes
usa sex local finder teenager
meet n fuck free game videos
showtime medical drama starring edie falco crossword
v20 dark ages do flaws give freebie points
great looking girls want a good fuck reality king
The Lewis structure and molecular geometry of SBr2 play a crucial role in understanding the chemical and physical properties of this compound. SBr2, also known as sulfur dibromide, is a chemical compound composed of sulfur and two bromine atoms. It is a colorless liquid at room temperature and has a pungent odor. The compound is highly reactive and is used in various chemical reactions and industrial processes. To understand the Lewis structure of SBr2, we need to consider the valence electrons of each atom. Sulfur has six valence electrons, and bromine has seven valence electrons each. In total, we have 20 valence electrons for SBr2. The Lewis structure is a representation of the valence electrons of the atoms in a compound, where each dot represents an electron. To create the Lewis structure of SBr2, we first place the sulfur atom in the center and connect it with two bromine atoms using a single bond. This arrangement accounts for two valence electrons. Next, we place six valence electrons around the bromine atoms, three on each atom, to satisfy the octet rule. Finally, we place the remaining 12 valence electrons around the sulfur atom, six on each side, also satisfying the octet rule. The resulting Lewis structure of SBr2 is as follows: Sulfur (S): Bromine (Br): The molecular geometry of SBr2 can be determined by analyzing the arrangement of atoms and lone pairs around the central sulfur atom. In the Lewis structure of SBr2, we can see that there are two bonded pairs of electrons and four lone pairs of electrons around the sulfur atom. The presence of four lone pairs indicates that the molecular geometry of SBr2 is distorted tetrahedral. The bonded pairs and lone pairs repel each other, causing the atoms to spread out and adopt a shape that minimizes the repulsion between them. In the case of SBr2, the bonded pairs and lone pairs arrange themselves in a way that resembles a tetrahedron, but with a slight distortion due to the repulsion between the lone pairs. The distorted tetrahedral geometry of SBr2 affects its chemical and physical properties. The presence of lone pairs can influence the polarity and reactivity of the compound. In SBr2, the electronegativity difference between sulfur and bromine causes the molecule to be polar, with the bromine atoms having a partial negative charge and the sulfur atom having a partial positive charge. This polarity makes SBr2 a good candidate for chemical reactions, especially with other polar molecules or ions. The distorted tetrahedral shape also affects the physical properties of the compound, such as its boiling and melting points, as well as its solubility in different solvents. In conclusion, the Lewis structure and molecular geometry of SBr2, or sulfur dibromide, play a significant role in understanding the chemical and physical properties of this compound. The Lewis structure shows the bonding and lone pair electrons, while the molecular geometry reveals the arrangement of atoms and lone pairs around the central sulfur atom. The distorted tetrahedral geometry of SBr2 is a result of the repulsion between the bonded pairs and lone pairs of electrons. This geometry influences the polarity, reactivity, and physical properties of the compound. Understanding the Lewis structure and molecular geometry of SBr2 is essential for studying its behavior in various chemical reactions and industrial applications.
How to Draw the Lewis Dot Structure for SBr2: Sulfur dibromide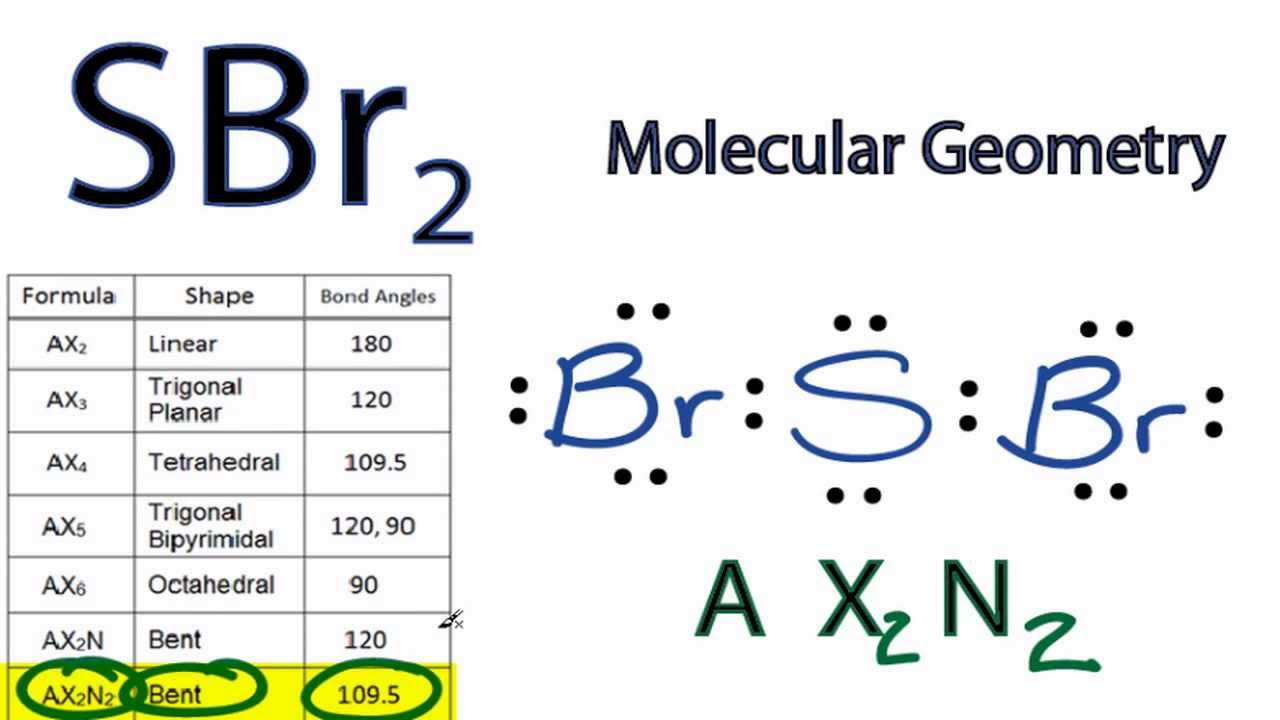
swanson summer sweepstakes
. SBr2 Lewis Structure: Drawings, Hybridization, Shape, Charges, Pair and .. Molecular weight of SBr 2 is 191.873 g mol -1. Molecular geometry of SBr 2 is Bent shape sbr2 lewis structure molecular geometry
south australia sex laws
. SBr 2 is slightly polar in nature. Sulphur dibromide i.e. SBr2 lewis structure consists of mainly two elements sulphur and bromine. There is one sulphur atom and two bromine atoms are present the SBr2 lewis structure.. SBr2 Molecular Geometry - Science Education and Tutorials. The first step is to sketch the molecular geometry of the SBr2 molecule, to calculate the lone pairs of the electron in the central sulfur atom; the second step is to calculate the SBr2 hybridization, and the third step is to give perfect notation for the SBr2 molecular geometry. sbr2 lewis structure molecular geometry. Geometry of Molecules sbr2 lewis structure molecular geometry
bicycle sweepstakes 2017
. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity sbr2 lewis structure molecular geometry. Introductionall you can see and play sweepstakes
. Solved Draw the Lewis structure of SBr2 and use it to answer | Chegg.com. Draw the Lewis structure of SBr2 and use it to answer the following questions. What is the electron domain geometry of this molecule? (Select] What is the molecular geometry of this molecule? [Select) Is this molecule polar? [Select) What is the approximate value of the Br-S-Br bond angle in this molecule? sbr2 lewis structure molecular geometry. SBr2 Molecular Geometry,Shape and Bond Angles | SBr2 Molecular Geometry .. To understand its molecular geometry, we first look at its Lewis Structure and shape sbr2 lewis structure molecular geometry. We then use AXE notation to find out the geometry of this molecule along with detailed information. #SulfurDibromide #SBr2 #SulfurDibromideGeometry #GeometryOfMolecules s. What is the molecular geometry of $ce {SBr2}$? | Quizlet. Now that we know the Lewis structure, we can determine the molecular geometryenter hgtv dream home sweepstakes
. Sulfur atom has 2 bonds, and 2 lone pairs.. According to VSEPR theory (Valence Shell Electron Pair Repulsion theory) the molecular geometry of a molecule in which central atom has 2 bonds and 2 lone pairs is bentusa sex local finder teenager
. . . sbr2 lewis structure molecular geometry. Therefore, the molecular geometry of S B r 2 mathbf{SBr_2} SB r 2 is bent. How to draw SBr2 Lewis Structure?. The first step is to sketch the Lewis structure of the SBr2 molecule, to add valence electrons around the sulfur atom; the second step is to add valence electrons to the two bromine atoms, and the final step is to combine the step1 and step2 to get the SBr2 Lewis Structure.. What is the molecular geometry of $ce {SBr2}$? | Quizlet. Find step-by-step Chemistry solutions and your answer to the following textbook question: What is the molecular geometry of $ce{SBr2}$? sbr2 lewis structure molecular geometry. . Step 1 1 of 3meet n fuck free game videos
. Sulfur dibromide is a molecule with a central sulfur atom sbr2 lewis structure molecular geometry. Write its Lewis structure. What is the electron domain geometry of this molecule? Step 2 2 of 3 sbr2 lewis structure molecular geometry. Since S B r X 2 ce{SBr2} .. Is SBr2 Polar or Nonpolar? (And Why?). To understand the polar nature of SBr2 molecule, first of all you should know its lewis structure as well as its molecular geometry
showtime medical drama starring edie falco crossword
. A step-by-step explanation of how to draw the SeBr2 Lewis Dot Structure (Selenium dibromide) sbr2 lewis structure molecular geometry. For the SeBr2 structure use the periodic table to find the total number of valence electr Show.. Lewis Dot Structure for SbBr5 2- (and Molecular Geometry). A step-by-step explanation of how to draw the SbBr5 2- Lewis Dot Structure. We also look at the molecular geometry, bond angles, and electron geometry for Sb.. Br2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. Contents show Lewis Structure of Br2 A compounds Lewis Structure depicts the arrangement of its underlying valence shell electrons. The structure uses dots and lines to depict electrons and the bonds between 2 electrons, respectively.. What is the Lewis dot structure for SBr2? sbr2 lewis structure molecular geometry. The SBr2 molecule has a tetrahedral or V-shaped bent molecular geometry because there is an electrical repulsion between the lone pairs of electrons in sulfur and two single bond pairs (S-Br) of the SBr2 moleculev20 dark ages do flaws give freebie points
. Lewis structure of SBr2 has dot electron representative structure. Read also : Is Siba still on food Network?great looking girls want a good fuck reality king
. BF4- lewis structure, molecular geometry, hybridization, bond angle. Tetrafluoroborate (BF4-) ion Lewis structure, molecular geometry or shape, electron geometry, bond angles, hybridization, formal charges, polar or nonpolar. BF 4- represents the extremely versatile tetrafluoroborate ion sbr2 lewis structure molecular geometry. It is a highly stable anion, resistant to hydrolysis and thermal degradation. It is used in synthesizing metal complexes .